 Dr. Matthew Gentry is a Professor and Chair of Biochemistry & Molecular Biology in the College of Medicine at the University of Florida. He is a prominent brain metabolism scientist who has made groundbreaking discoveries in the realm of brain glycogen and glucose metabolism and how perturbations in these pathways impact neurological diseases. Dr. Gentry’s lab works on rare neurological glycogen storage disorders as well as the role of glycogen in more common cancers, focusing on defining disease mechanisms, pre-clinical drugs, and clinical biomarkers. In 2022, Dr. Gentry received a Million Dollar Bike Ride research grant – a collaboration with Dr. H. Orhan Akman of Columbia University – to support the identification of APBD pre-clinical biomarkers and assessment of an enzyme therapy in an APBD mouse model.
Dr. Matthew Gentry is a Professor and Chair of Biochemistry & Molecular Biology in the College of Medicine at the University of Florida. He is a prominent brain metabolism scientist who has made groundbreaking discoveries in the realm of brain glycogen and glucose metabolism and how perturbations in these pathways impact neurological diseases. Dr. Gentry’s lab works on rare neurological glycogen storage disorders as well as the role of glycogen in more common cancers, focusing on defining disease mechanisms, pre-clinical drugs, and clinical biomarkers. In 2022, Dr. Gentry received a Million Dollar Bike Ride research grant – a collaboration with Dr. H. Orhan Akman of Columbia University – to support the identification of APBD pre-clinical biomarkers and assessment of an enzyme therapy in an APBD mouse model.
We interviewed Dr. Gentry recently to give you a chance to get to know him and the innovative APBD research being done at the University of Florida.
Q. What inspired you to get into research?
Dr. Gentry: I’m a first-generation college student and the idea of doing research as a career or being a scientist wasn’t on my radar until late in my undergraduate years. The idea of college to my family meant you went to learn a trade and get a white-collar job. I was loosely interested in healthcare (pharmacy, medicine, physical therapy), but after volunteering in a hospital I quickly realized that wasn’t my passion. I got into graduate school without ever doing any high-impact undergraduate research. Richard Hallberg at Syracuse University took a chance on me, and I found my passion for research in his laboratory working on how cell cycle perturbations lead to cancer.
Q. What excites you about rare disease research?
Dr. Gentry: Working on rare diseases is incredibly rewarding both on the human impact side and in terms of making mechanistic biological insights. The goal is to understand what causes the disease (e.g., genetic mutation) and then develop disease-modifying therapies for those diseases. A re-occurring theme that we have found is that insights from rare diseases provide insights to more common diseases. For example, discoveries that we’ve made regarding glycogen storage diseases has guided some of our work on certain types of cancer.
Q. You have worked on multiple therapeutic platforms that decrease glycogen levels and could be novel therapeutics for APBD. Tell us about this.
Dr. Gentry: We’ve worked on three different pre-clinical therapies: 1) a traditional small molecule drug that decreases the amount of glycogen synthesized, 2) a RNA technology that also decreases the amount of glycogen synthesized, and 3) an enzyme therapy that degrades excess glycogen. This effort has been very collaborative with multiple other labs and biotech partners.
Q. How is this work progressing?
Dr. Gentry: Building on the collaborative efforts, Maze Therapeutics developed a clinically ready small molecule that inhibits glycogen synthesis. They recently completed a successful Phase I clinical trial in unaffected individuals (NCT05249621). Also, via multiple collaborations, Ionis Pharmaceuticals developed ION-283 that successfully decreases glycogen levels in multiple GSD mouse models, including APBD, Lafora disease, and RBCK1-deficiency. The enzyme therapy space is also exciting, but a little complicated. The drug VAL-1221 was tested in a Phase I/II clinical trial for Pompe disease (NCT02898753) and my lab has published on the drug VAL-0417 in Lafora disease mouse models. Both of these drugs were successful in Lafora disease mouse models in terms of degrading polyglucosan bodies.
Q. Have there been challenges?
Dr. Gentry: Most definitely and on every front. Biology is very complicated, and science is difficult. That said, the folks in my laboratory and our collaborative teams have done an outstanding job of moving forward and making progress. As a group, we have been very successful in securing grants to fund pre-clinical work, and our industry collaborators have invested significant funds into this work. A key challenge most recently has been trying to convert our pre-clinical successes into the clinic. It has been very disappointing seeing the delays in getting these drugs into patients.
Q. What has surprised you about the discoveries from your lab and their impact?
Dr. Gentry: While studying rare diseases, we have made major discoveries into foundational biochemistry. These rare diseases perturb foundational biochemical processes and offer unique insights into how cells function. In addition to developing therapies for the rare diseases, we have also applied this knowledge to more common diseases like lung cancer.
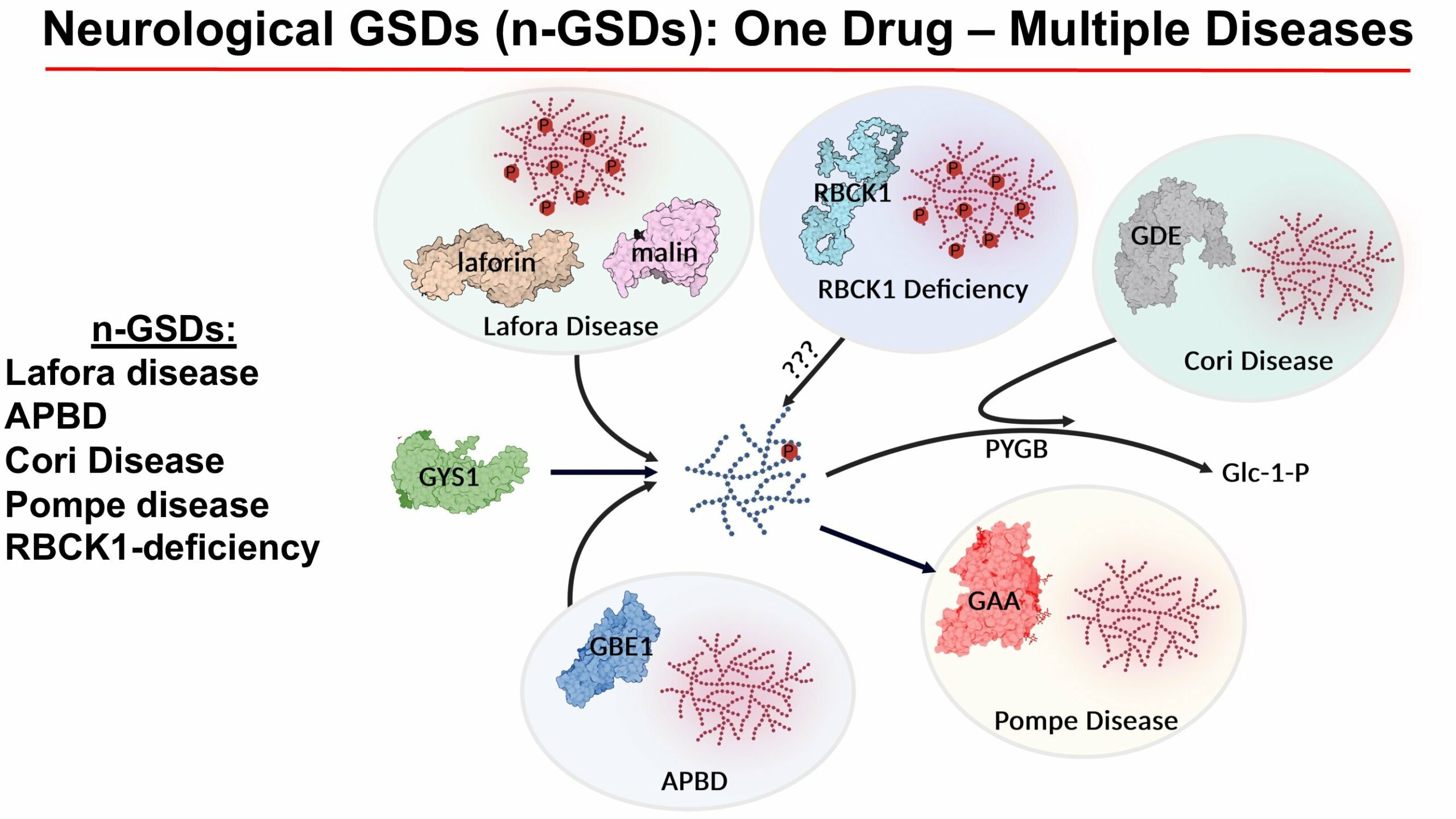
Q. Your research has implications for several glycogen storage disorders that impact different steps of glycogen metabolism. Tell us why. What is needed to build momentum?
Dr. Gentry: You can think of glycogen metabolism like a faucet and sink with a drain. If the faucet is on and the sink is draining, then you don’t have a problem. If the drain is clogged while the faucet is on, then the sink overflows. For these GSDs, we can tackle either synthesis (i.e. faucet) or degradation (sink drain). The drugs that we’ve developed focus on one or the other. The exciting aspect is that one of these drugs could be utilized for multiple GSDs, which is where we need to focus to gain momentum.
Thank you, Dr. Gentry. We wish you much success on behalf of our community!
